This information is for general purposes only and should not be considered as medical advice. Always consult with a qualified healthcare professional for any medical concerns or questions you may have.
Milistan Multisymptomic: Relief for Multiple Ailments
Milistan Multisymptomic is a powerful medicine that offers relief from a variety of symptoms. Each pack contains 12 tablets, providing fast and effective relief. Below, we'll explore the ingredients and composition, indications and uses, precautions and side effects, and conclude with important information about this medication.
Ingredients and Composition
Milistan Multisymptomic contains a unique blend of active ingredients that work together to provide relief from multiple symptoms. The composition includes pain relievers, fever reducers, decongestants, antihistamines, and cough suppressants. These ingredients work synergistically to provide comprehensive relief from a range of ailments.
Indications and Uses
This medication is indicated for the relief of symptoms associated with colds, flu, allergies, and other respiratory conditions. It provides relief from nasal congestion, sinus pressure, cough, sore throat, and fever. Milistan Multisymptomic is ideal for individuals seeking relief from multiple symptoms without the need for multiple medications.
Precautions and Side Effects
It's important to follow the recommended dosage and usage instructions to avoid potential side effects. Common side effects may include drowsiness, dizziness, dry mouth, and nausea. It's important to consult with a healthcare professional before using this medication, especially for individuals with pre-existing medical conditions or those taking other medications.
Conclusion
Milistan Multisymptomic is an effective and convenient solution for individuals seeking relief from multiple symptoms. Its unique combination of ingredients offers comprehensive relief from cold, flu, allergies, and respiratory conditions. However, it's important to use this medication as directed and consult with a healthcare professional if you have any concerns or questions.
Note: This article is for informational purposes only and should not be considered medical advice.Additional Information
| SKU | m135 |
|---|---|
| Brand | No |
| Size | No |
| Manufacturer | No |
- Be the first to review this product
Write Your Own Review
Products on sale
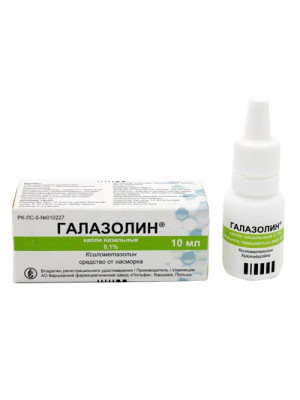
GALAZOLIN NASAL DROPS 0.1%, 10ML
Regular Price: $10.99
Special Price $8.99
Activated Charcoal #10
Regular Price: $3.99
Special Price $2.50
ACC 200 powder for preparing an oral solution Orange 3g No. 20
Regular Price: $24.59
Special Price $18.99
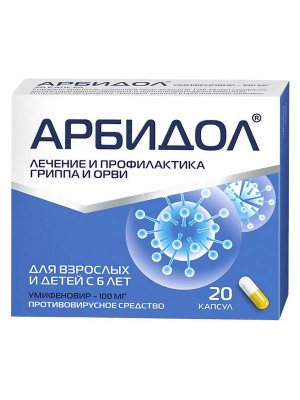
Arbidol capsules 100 mg No. 20
Regular Price: $27.99
Special Price $24.99
Valerian Tincture 25ml
Regular Price: $6.99
Special Price $5.99
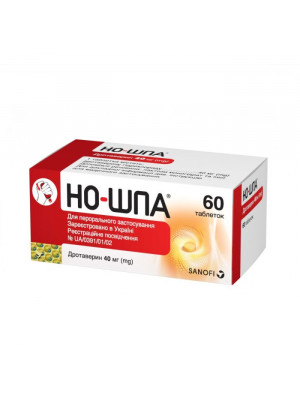
No-Spa ( Noshpa ) no spa 40mg №60
Regular Price: $15.99
Special Price $14.49
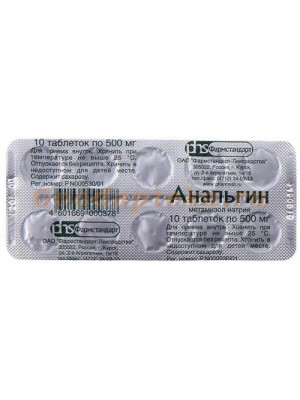
Analgin 10 Tablets
Regular Price: $5.99
Special Price $4.78
Also Purchased